Exactly what is kinetic molecular theory definition ? kinetic theory, A theory of the thermodynamic behavior of issue, specifically the relationships amongst stress, quantity, and temperature in gases, based upon the dependence of temperature level on the kinetic energy of the quickly moving bits of a material. The theory makes use of statistical auto mechanics under the assumption that energy and momentum are conserved in all collisions between fragments.
What is The Kinetic Molecular Theory?

youtube.com
The experimental observations about the habits of gases reviewed so far could be clarified with a straightforward theoretical model called the kinetic molecular theory. This theory is based on the following proposes, or assumptions.
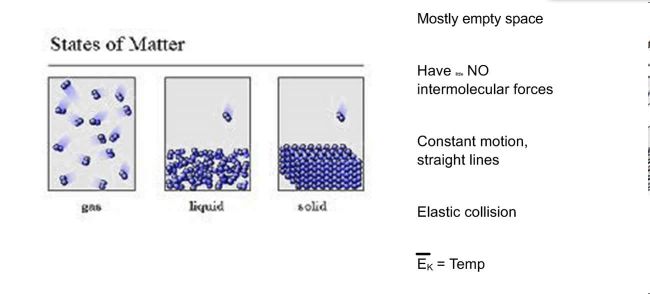
- Gases are made up of a large number of fragments that behave like hard, round objects in a state of consistent, arbitrary motion.
- These fragments move in a straight line up until they collide with one more bit or the walls of the container.
- These bits are a lot smaller sized than the range in between particles. The majority of the volume of a gas is therefore void.
- There is no force of attraction between gas bits or in between the bits as well as the walls of the container.
- Collisions in between gas particles or collisions with the wall surfaces of the container are perfectly elastic. None of the energy of a gas particle is shed when it hits one more particle or with the walls of the container.
- The average kinetic power of a collection of gas fragments depends on the temperature level of the gas as well as nothing else.
The presumptions behind the kinetic molecular theory could be illustrated with the apparatus shown in the figure below, which contains a glass plate surrounded by walls placed in addition to 3 vibrating electric motors. A handful of steel round bearings are placed on top of the glass plate to stand for the gas fragments.
When the electric motors are switched on, the glass plate vibrates, which makes the sphere bearings relocate a consistent, random style (propose 1). Each sphere relocates a straight line up until it hits another round or with the wall surfaces of the container (postulate 2). Although crashes are regular, the ordinary distance in between the round bearings is a lot bigger than the size of the rounds (postulate 3). There is no pressure of destination in between the specific ball bearings or between the sphere bearings and the walls of the container (postulate 4).
The crashes that occur in this apparatus are extremely various from those that happen when a rubber ball is dropped on the flooring. Collisions between the rubber sphere as well as the flooring are inelastic, as shown in the figure below. A part of the power of the ball is shed each time it strikes the flooring, up until it ultimately rolls to a stop. In this apparatus, the collisions are perfectly flexible. The spheres have equally as much power after a crash as before (postulate 5).
Any things moving has a kinetic power that is specified as half of the product of its mass times its speed settled.
KE = 1/2 mv2
At any moment, several of the round bearings on this device are relocating faster compared to others, however the system can be defined by an average kinetic power. When we boost the “temperature” of the system by raising the voltage to the electric motors, we find that the typical kinetic power of the ball bearings increases (postulate 6).
Assumptions Kinetic Molecular Theory
youtube.com
The complying with are the assumptions of kinetic theory of gas which are additionally called postulates of Kinetic theory of gas:
( i) Assumptions Regarding the Molecules
- Every gas contains incredibly little particles known as particles. The particles of an offered gas are all the same however are various than those an additional gas.
- The molecules of a gas are identical, round, rigid and also flawlessly flexible point masses.
- Their size is minimal in comparison to inter molecular distance (10-9 m).
( ii) Assumptions Pertaining to Quantity
- The Quantity of particles is negligible in contrast to the volume of gas. (The quantity of molecules is only 0.014 % of the quantity of gas).
( iii) Assumptions Regarding Motion:
- Molecules of a gas go on relocating randomly in all possible instructions with all possible speeds.
- The speed of a gas particle lies between no as well as infinity (extremely high speed).
- The variety of particles relocating with many possible rate is maximum.
( iv) Assumptions Concerning Crash:.
- The gas molecules go on colliding amongst themselves too with the walls of the containing vessel. These crashes are perfectly elastic. (That is the complete power prior to the accident is equal to the overall power after the accident).
- Particles move in a straight line with consistent speed throughout succeeding collisions.
- The range covered by the molecules in between two succeeding collisions is referred to as totally free path and mean of all the totally free courses is referred to as Mean Free Path.
- The moment spent in a collision in between 2 molecules is negligible in comparison to time between 2 successive collisions.
- If we take into consideration the number of crashes present in one unit quantity after that the number stays continuous.
( v) Assumptions Pertaining to the Pressure:.
- No eye-catching or repulsive pressure acts between gas molecules.
- Gravitational tourist attraction among the molecules is ineffective because of incredibly tiny mass and also really broadband of molecules.
( vi) Assumptions Regarding Stress:.
- Particles constantly ram the wall surfaces of container as a result of which their energy adjustments.
- This modification in momentum is transferred to the walls of the container.
- Consequently stress is exerted by gas molecules on the wall surfaces of container.
( vii) Assumptions Regarding the Thickness:.
- The density of a gas is continuous at all points of the container.
Why is the Kinetic Molecular Theory Important?

Why is the Kinetic Molecular Theory Important?
It is the only theory we have that precisely predicts macroscopic buildings like temperature, stress, and thermal growth.
The kinetic molecular theory supplies physicist with a device to comprehend things like temperature level and pressure. This theory allows researchers to understand what it means for something to be hot or cold, how warmth is transferred from one material to one more, as well as why the temperature level, stress, and also quantity of a gas are all related.
These concepts are in charge of the production of the burning engine, the refrigerator, heat pumps, as well as unique products made use of to enhance or lower warmth transfer.
Presently, a great deal of research is being put right into energy storage solutions. This is an important step before tidy power sources like wind as well as solar can replace unclean carbon based energy sources like coal as well as oil. Compressed gas storage is among the leading feasible solutions to long term power storage space; and kinetic molecular theory is the essential physics behind this service.
Kinetic Molecular Theory of Matter
amazonaws.com/
The kinetic theory of issue was used by the scientist to explain the habits of the atoms and molecules in solids, fluids as well as gases.
- The Kinetic molecular theory considers that atoms and particles making up the issue are always in consistent motion.
- This theory was able to explain the forces that engage in between the molecules or fragments in all the three state of issue.
- The kinetic molecular theory of matter likewise takes the temperature of the compound as well as is basically as a result of the power of the atoms or molecules which constitute the issue.
- When the temperature level of the issue is increased after that the energy possessed by the particles rises and thus they relocate quicker, that is the kinetic power increases.
- It can be stated that the temperature is made use of to determine the energy of the movement in between the particles.
Kinetic Theory of Gases Proposes
ThoughtCo.com
The fundamental principle of the kinetic theory of gases was provided by Daniel Bernoulli. Then several writers have actually contributed to the theory.Clausius as well as Maxwell are leaders in the development of the modern-day kinetic theory of gases.
The kinetic molecular theory is clarified by the following postulates which define the habits of gas particles (that is the habits of the molecules as well as atoms of gases).
- Gases are thought about as containing a multitude of particles which are called as atoms or molecules. Each atoms or particles behave like a completely similar elastic round. Essentially this propose takes into consideration atoms of gases as similar with no distinction in the form or ise and also as elastic round.
- The particles or particles of the gas are in continuous motion. The movement of the molecules or atoms of the gases are likewise taken into consideration to be arbitrary and they relocate all instructions. If the gas molecules do not move after that they occupy a repaired position and also they will have a repaired shape. But it is understood that gases use up the form of the container.
- The size of the each particle constituting the gas bulk is considered to be very small; the distance between the particles of the gas is really high compared to the quantity of the particle. Thus the actual volume inhabited by the molecules of the gas is extremely negligible in contrast to the area in between the particles of the gases. The gas molecules are thought about to be point mass. That is their quantity is very much less as compared to the evident volume. So the quantity of the gas particles is considered to be zero. This postulate of the gas clarifies its high compressibility.
- The particles of gas do not bring in an additional molecule. That is the forces of attraction or forces of repulsion between the gas particles are extremely less. The molecules are not attracted in the direction of the walls of the container. This postulate describes why gases could broaden, because the particle has no tourist attraction between them they can broaden when the volume of the container is increased and also occupy the space which is offered.
- Gases are taken into consideration to move in all instructions along a straight line. During their movement they stumble upon each other. They colloid with each other as well as the collision of the particles of the gas are taken into consideration to be completely flexible. They additionally colloid versus the wall of the container. The accident with each other and also with the walls is flawlessly elastic. Their momentum and kinetic energy does not alter during the accident, although the energy as well as the kinetic energy can be transferred from one molecule to an additional.
- Particles move along the straight line throughout the accident. The typical range traveled by the particles throughout the two succeeding collisions is called the mean free course of the colliding particles.
- The pressure of the gas is because of the collision of the particles in the walls of the container. The pressure is referred to as the force each location. When the gas molecules inhabit a smaller quantity after that the area would certainly be reduced and also the force each location would certainly be big. So the pressure is high.
Kinetic Molecular Theory of Gases
The Molecular Theory of Matter is a prediction that exactly how matter must behave, based on some assesments and estimations.
- The theoretical assesments are only monitorings videotaped from experiments.
- There are examples where we examine that materials include tiny molecules or atoms.
- Estimations are made to keep these sort of academic descriptions very straightforward.
Matter consists of small Fragments
The to start with presumption is that all matter contains of lot of minute particles as well as are mainly private atoms or molecules.
Big Separation In Between Fragments
The following assumption is worried concerning the splitting up of the fragments.
In an aeriform tool, the room in between the bits are very large when compared to their dimension, resulting in the absence of any type of appealing or undesirable pressures in between the molecules.
In a liquid medium, although the range in between the particles is large, but they are close enough to permit the attractive forces to constrain the material to a particular form of its container.
In a strong, the bits are packed extremely close and the pressures of tourist attraction are confined to the product itself and also this aids in maintaining a certain shape.
Bits In Continuous Movement
The following assumption is that each particle remains in constant motion.
In gaseous medium, the bit motion is located to be extremely random as well as with no details or pre-destined instructions.
In fluid medium, the particle motion is restricted to a huge extent by the quantity of the liquid.
In solids, the fragments motion is entirely or practically restricted within a little area, which helps the solid to preserve its shape.
The kinetic energy is primarily figured out by the rate of each getting involved particle.
Kinetic Molecular Model
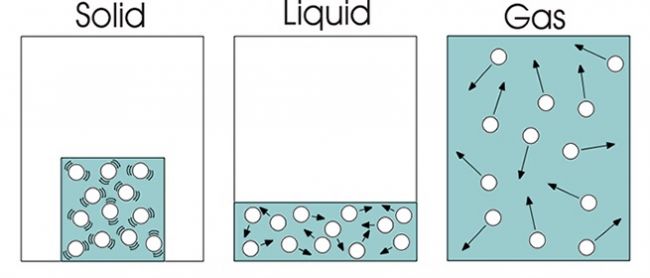
Kinetic Molecular Model
The Molecular Theory of Matter states that “matter comprises of a large number of small bits which are in continuous motion”. This theory likewise presumes that bits are little and also widely divided. They ram each various other and exchange energy.
The theory helps in clarifying the flow or transfer of warm as well as the relationship between pressure, temperature as well as the quantity homes of gases.
Kinetic Molecular Theory as well as Temperature Level
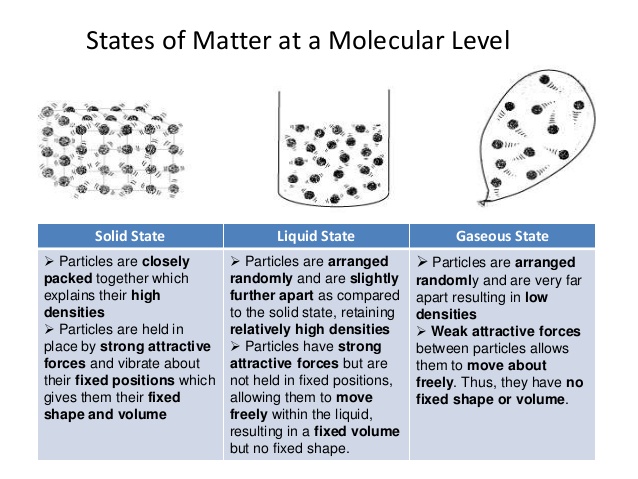
slidesharecdn.com
All particles of a gas do not have the very same rate, and this could be described by the truth that throughout the collision of molecules, the rate or energy adjustments. If at first the rate of a molecule is the same, it transforms after the molecular accident.
So it is seen that fragments have different rates, which are changing regularly. Yet although specific speed changes, the distribution of rate continues to be constant. That is the complete speed that does not change. Yet if the temperature of a gas is boosted, after that the average kinetic power likewise changes.
Due to the fact that the particles of the gas relocates with better speed, the power of the molecule changes therefore of the warmth put on the gas system. So the typical kinetic energy increases and also the rate distribution boosts.
Originally posted 2020-01-07 02:27:45.